
Scarborough manufacturer's device may help more COVID-19 patients breathe
 Courtesy / Amplify Additive
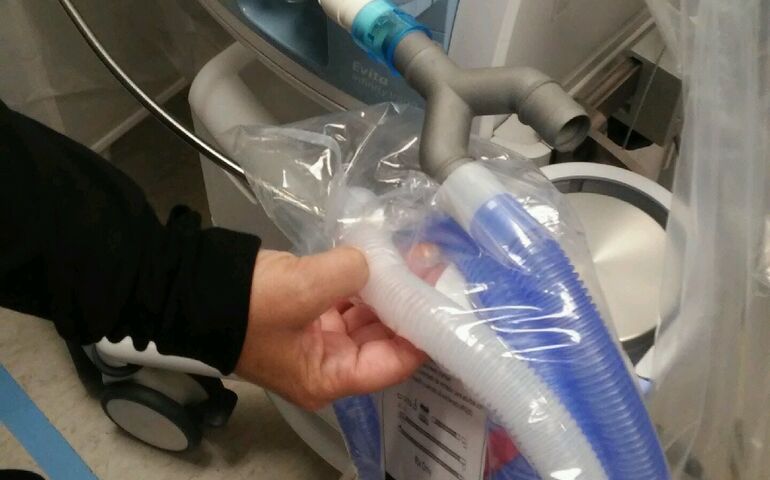
A trial version of Amplify Additive’s ventilator adapter is shown here at Huston Medical Center in Kansas.
Courtesy / Amplify Additive
A trial version of Amplify Additive’s ventilator adapter is shown here at Huston Medical Center in Kansas.
A Scarborough company, Amplify Additive, is developing a device to make more efficient use of scarce medical ventilators, desperately needed to treat patients with COVID-19.
Where enough ventilators aren't available, Amplify's splitter device might allow one ventilator to help multiple patients breathe.
Brian McLaughlin, the company’s founder and owner, and his team scoured the internet for open-source designs of products that could help alleviate the current shortage of lifesaving equipment, according to a news release.
The team accessed a design from Interactive CAD Solutions for a ventilator splitter and adapted it to their software and 3D printing machines. Amplify began working on titanium-printed prototypes a week ago.
“We can manufacture 40 at a time on each machine and we have three machines, so 120 parts at a time,” McLaughlin told Mainebiz. “The material is implant-grade titanium that is used for orthopedic implant applications, so these can be sterilized in the hospital setting via autoclaves.”

Amplify Additive uses a type of additive manufacturing — commonly called 3D printing — in which electron beams fuse successive layers of titanium powder in a vacuum to create medical implants. The process is called “electron beam melting” or EBM. Parts are printed in 50-micron layers, about the thickness of a human hair.
Since receiving the CAD file about 10 days ago, turnover has been quick, McLaughlin told Mainebiz. He printed multiple prototypes of a three-to-one and a two-to-one splitter over the weekend and determined the two-to-one best fit his company’s manufacturing capability.
After printing and finishing titanium versions the following Monday, he shipped them Tuesday for testing at F.W. Huston Medical Center in Winchester, Kan., a hospital he's worked with in the past. The center made design recommendations and Amplify modified the splitters accordingly.
A new version was expected to ship to Huston this week. Amplify is now looking into getting approvals from the federal Food and Drug Administration.
“We are essentially situated to address this a timely manner,” McLaughlin said.










0 Comments